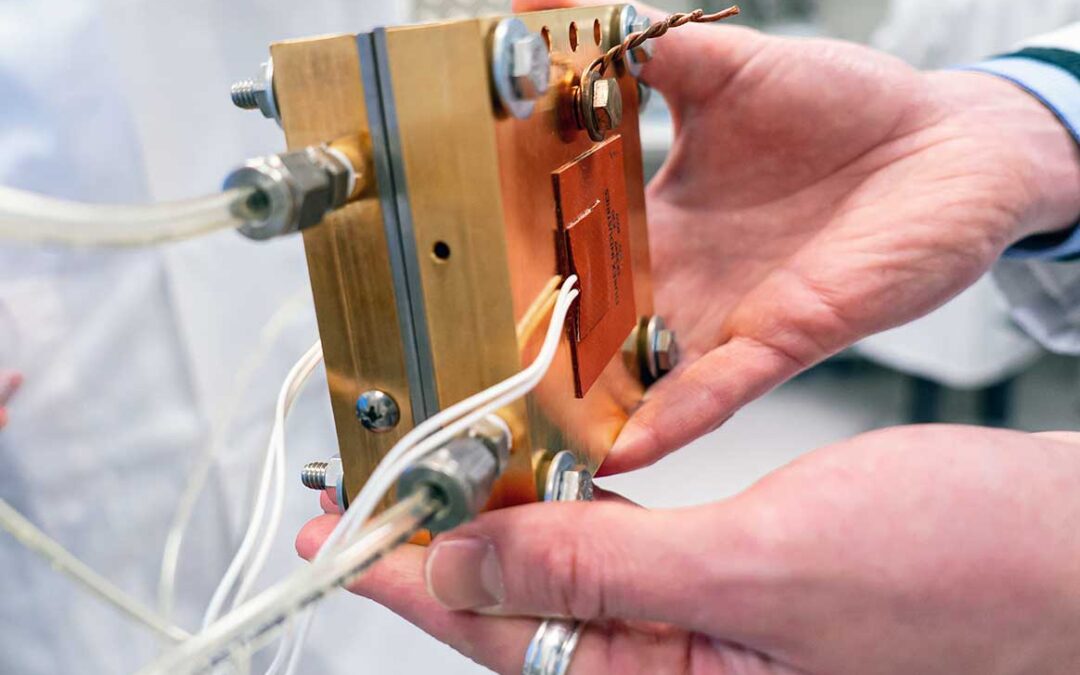
Bio-based membrane technologies developed by the Digital Cellulose Center have laid the foundation for the startup company Cellfion. In the future, their nanocellulose membranes will enable the fabrication of renewable energy storage devices, like hydrogen fuel cells and redox flow batteries, as well as replace the current non-renewable membranes on the market.
The bio-based membranes from Cellfion are made of nanocellulose fibrils, a renewable material extracted from wood. The ion-selective membrane will be used in large-scale energy storage applications like hydrogen fuel cells or redox flow batteries. The research behind Cellfion’s membrane originates from the Digital Cellulose Center (DCC) research on Ion-selective membranes.
“Cellfion is a great example of how the dual excellence and multidisciplinary of research centers like the DCC can fast-track the journey from fundamental research to commercialization by having a close collaboration between industry and academia”, says Jesper Edberg, researcher at RISE and scientific leader at the Digital Cellulose Center. “We are also proud that the first Digital Cellulose Center Ph.D. student to graduate, Johan Erlandsson, is now the CTO of Cellfion.”

Membrane for hydrogen fuel cells and redox flow batteries
Membranes are a key component in energy storage devices, and they are often the part that first breaks down and needs to be replaced. Cellfion manufactures nanocellulose membranes for electrochemical energy storage and conversion devices, mainly focusing on two applications: organic redox flow batteries, a form of large-scale energy storage that can be used to support the electricity grid, and PEM electrolyzers, which are used to produce green hydrogen gas.
The customer-specific bio-based membrane
The startup company Cellfion is based in Norrköping, Sweden, and was founded in 2021. Cellfion’s CEO, Liam Hardey, explains that their goal is to offer the first commercial bio-based ion-selective membranes for clean energy devices. Developing the membrane is the first step in creating an organic redox flow battery. He continues:
“The properties of Cellfion’s membranes can be tuned to suit specific demands and be tailor-made for specific applications. The wide range of chemicals available for redox flow batteries have different requirements on the membrane. During our market research businesses said that there were not enough options for ion membranes that they can use for their specific product. Our competitors deliver standard products, whereas we can tune our solution to the customer’s needs which can lead to an increase in performance and the lowering of costs.”

The current commercial membranes on the market are high-cost and usually fossil-based. Some contain controversial forever-chemicals – Perfluoroalkyl substances (PFAS), which the EU has decided to ban in phases, starting 2023.
“If the clean energy industry is to become truly sustainable, we need to ensure that the materials we are using are also that,” says Liam.”
New pilot facility for prototyping bio-based membranes
“We have demonstrated that the membrane performs well in certain conditions in a lab environment, having cycled batteries for more than 500 cycles without seeing signs of degradation,” says Liam Hardey. “The next step is to implement our technology into relevant industry environments and to begin tuning the membrane to specific systems with the aim of increasing efficiencies.”
He continues:
“We are currently in the process of taking in our next capital injection to the company which will enable us to build our first pilot facility for rapid prototyping of our material to send to customers and begin validating the technology externally. We have quite an aggressive timeline ahead of us where we aim to begin scaling the technology during 2023 to be able to meet the market demand and growth.”
This is how membranes work
An ion-selective membrane is a central component found in many electrochemical devices such as fuel cells, redox flow batteries, and electrolyzers. The membrane is often referred to as the heart of the cell and is most importantly a major component in defining the performance of an electrochemical device.
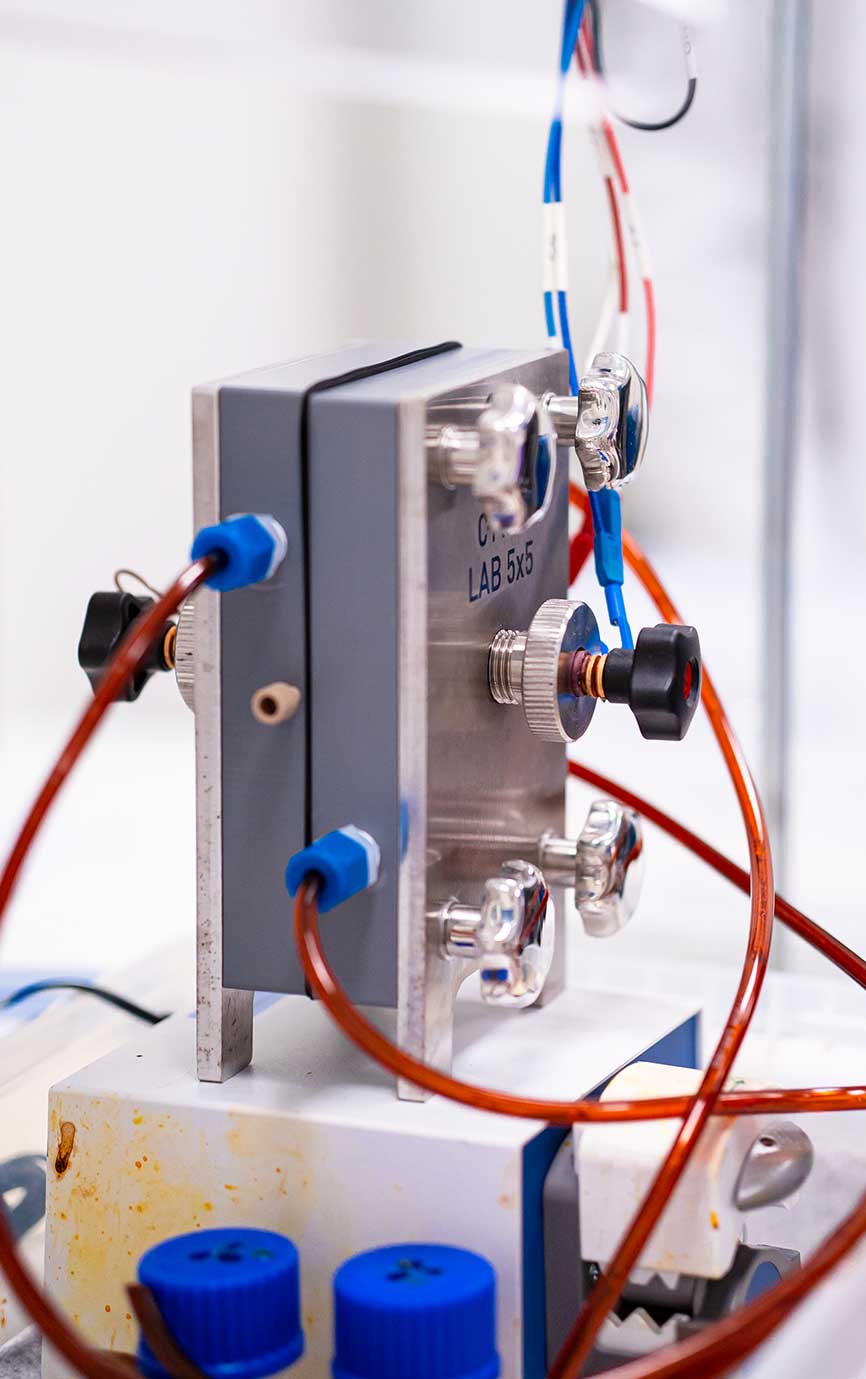
The membrane is usually integrated between two electrodes (anode and cathode). This allows for only specific selected ions to be transported across it, whilst at the same time, blocking the unwanted molecules. Moreover, an ion-selective membrane allows for specific ion selection through chemical modifications and size excursion created by the pores in the membrane.